Black Phosphorous-Based Materials for Energy Applications
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2878 words | ✅ Published: 11 Oct 2021 |
EXECUTIVE SUMMARY
Black Phosphorous (BP) comes under the family of two-dimensional materials, which include materials such as graphene, transition metal dichalcogenides (TMDs), silicene, hexagonal boron nitride and MXenes. In 2014, bulk BP was first mechanically exfoliated to produce monolayer and few layers phosphorene. Since its inception, it has garnered a lot of interest among the researchers due to its superior electronic, mechanical, optical and thermal properties and its anisotropic puckered structure. Some great advantages of BP over other 2D materials are, the tunable band gap (0.3 to 2eV), which is highly dependent on the layer number, high carrier mobility (~1000 cm2V-1s-1), enhanced in-plane anisotropy and a high specific surface area (SSA). Control over these properties is an important step towards using BP in various applications in electronic, energy and biomedical industries.
However, BP suffers from several problems such as instability in ambient conditions if exposed for a long time, large volume change upon charging/discharging, which can significantly degrade the properties. Another challenge that BP faces is the lack of low cost synthesis techniques, which can be used for mass production of BP-based devices. In spite of these challenges, current research in BP-based materials is focused heavily on improving the performance of the devices, overcoming the instability problem using passivation and functionalization techniques and manipulating the process parameters for low cost, high quality material synthesis.
This paper discusses several strategies that can be employed for improving the performance of energy storage devices such as supercapacitors, batteries and integration of BP in energy generation devices such as fuel cells as well as other miscellaneous applications in H2 storage and solar water steam generation.
A common theme that the paper adopts for most of the applications is the optimization and increase in SSAs of the phosphorene sheets. Some suggested strategies include, tuning the layer number, inducing disorder in the nanosheets and addition of conductive metal oxide particles in optimal amount on a BP substrate. All these strategies have been found to be successful according to the literature of other 2D materials. Another area which has been relatively unexplored in the literature is the understanding of pore chemistry, pore size and morphology, which has been suggested in the paper.
A new application of BP, which has been suggested, is in the field of microbial and enzymatic bio-fuel cells. These fuel cells depend on the energy generated from the microorganisms and enzymatic reactions involving glucose inside the human bodies. Also, using BP in H2 storage and solar water steam generation technologies can significantly reduce the cost of energy generation and improve the efficiency of the system.
Implementation of the suggested ideas would benefit the initiative towards cleaner energy technologies, while keeping the performance of the devices at-par with the existing technologies at same or a lower cost. Therefore, these ideas will be a stepping stone towards commercialization of BP-based technologies in energy sector and beyond.
INTRODUCTION
Since the discovery of graphene in 2004, extensive research has been carried out in the field of two-dimensional (2D) materials, and they have emerged as a popular choice for a number of applications in the fields of electronics, energy storage and generation, and biology. Among the vast list of 2D materials, which include transition metal dichalcogenides (TMDs), silicene, hexagonal boron nitride and MXenes, black phosphorous (BP) has emerged as a rising star, owing to its excellent properties in terms of a tunable direct band-gap, high theoretical specific capacity, high carrier mobility and an anisotropic structure [1,2].
Black phosphorous in bulk form has been around for the last 100 years, but it was only in 2014, when monolayer or few layer black phosphorous was successfully mechanically exfoliated [3]. This 2D form of BP is popularly called as phosphorene. Since its discovery, a considerable amount of theoretical and experimental research has been carried out to explore the electronic, structural, optical and thermal properties for potential applications.
Phosphorene has a layered structure, much like graphene, with stacked layers, held together by weak Van der Waals forces and in-plane covalent bonding with P sharing bonds with 3 other P atoms. However, unlike graphene which has a planar structure, a monolayer of BP has a puckered honeycomb structure with strong in-plane anisotropy which gives rise to marvelous electronic transport and strain properties [4], with applications in thin-film and photo-electronics. Another unique property of BP is the ability to control the number of layers in the structure, allowing for a tunable band gap (around 0.3eV for thick BP and 2eV for monolayer BP) [2]. The large tunable band-gap range of BP covers a lot of the electromagnetic spectrum from infrared to visible light. Thus, BP can be used in thermoelectrics, fiber optics, photovoltaics and thermal imaging, giving it a significant advantage over zero band-gap graphene and high band-gap TMDs (~1.8eV).
BP has shown high carrier mobility (~1000 cm2V-1s-1) and a large on/off ratio (upto 104 at room temperature) [5], making it viable to be used in field-effect transistor (FET) devices. In terms of its applications in energy storage, BP has been used in supercapacitors, lithium and sodium ion batteries (LIBs and SIBs) due to its large theoretical specific capacity (2596 mAh g-1) [6] over the traditional graphite electrode (372 mAh g-1) and a preferred Li and Na ion diffusion in the zig-zag direction [7].
Even though BP has a good potential to replace the existing materials in energy generation and electronics, it suffers from some problems which have hindered its scalability into commercial applications. 2D BP is unstable in ambient conditions, as it reacts with air, oxygen and water vapor and degrades substantially, leading to deterioration in the electronic, optical and mechanical properties. Current state-of-the-art strategies that are being used to overcome these problems are surface passivation, encapsulation, functionalization with polymer ligands and doping with other metals. Also, the synthesis techniques used for BP are not cost-effective and are difficult to scale for mass-production.
This paper talks about different strategies that can be used to improve the performance of the BP for supercapacitors and battery applications. In addition, unexplored applications of BP in the field of fuel cells, hydrogen storage and adsorption and solar water steam generation will be investigated.
IMPROVED AND NEW APPROACH TO ENERGY APPLICATIONS OF BLACK PHOSPHOROUS
Supercapacitors (SCs)
Supercapacitors are important energy storage devices, which are known for their high power density, excellent cycle stability and ultrafast charging/discharging ability [8], as they store electrical charges in the ion-double layer at the electrode/electrolyte interface. Over the years, 2D materials such as graphene, carbon nanotubes [9] and TMDs have been used to fabricate SC electrodes. Recently, liquid exfoliated BP nanoflakes with acetone have been used to fabricate a high performance flexible supercapacitor [10] shown in Fig.1 and the performance is shown in Fig.2


Fig.1 Fabrication of BP flexible supercapacitor [10] Fig.2 Cycle stability of the BP supercapacitor
One approach that can be adopted for improving the performance is the tuning of the layer number of the 2D BP. It is known that the capacitance is dependent on the specific surface area (SSA) of the electrodes. Thus, using a multilayer BP means increased SSA and thus increased performance. Also, BP hybrid materials can be used in terms of nanocomposites and incorporation of metal oxides such as ZnO and SnO2 into the BP lattice can lead to an increase in capacitive performance as seen in the case of graphene [11]. Similarly, mixing of metallic foam and conductive nanoparticles with BP can lead to an improved charge transfer and better specific capacitance. Metallic foam has a three-dimensional porous structure and adding it to BP in an optimal amount can affect the pore size and distribution.
Another approach that can be used is inducing disorder in the BP sheets. In some cases, disordered structures can exhibit superior properties such as high reversible capacity and good cyclic performance. Also, disorder in the structure increases the SSA and thus the specific capacitance. Research areas that can be explored for increasing SC performance are pore chemistry and morphology, optimal doping concentration of materials and tuning the structure of BP in order to maximize the SSA.
Batteries
Rechargeable Li and Na ion batteries with graphite anodes have been used for many years for commercial applications due to their stable cyclic performance and high energy density. However, significant research has been carried out into alternatives for graphite which has a limited capacity of 372 mAh g-1 with energy density of 200 mWh g-1 [12].
Recently, BP nanocomposite has been used in order to improve the battery performance due to its high theoretical specific capacity of 2596 mAh g-1. BP has been used in combination with graphite and graphene to form composite anodes which have delivered high charging and discharging capacities of 2000 and 1800 mAhg-1 respectively after 100 cycles [13]. However, phosphorous being a non-metal suffers from large volume change (~300%) during charging and discharging, thus reducing the cycle stability [14].
A strategy that can be employed is the incorporation of metal oxide nanoparticles such as Mn3O4 and Fe3O4. It has been seen in literature that combining 2D materials with Fe3O4, can provide improved electron pathway and thus, improved cycle stability and performance [15]. One of the reasons for this improvement can be that, the BP could provide a large contact area for the dispersion of the Fe3O4 particles and thus improve the electron transport. Also, Fe3O4 can prevent restacking of BP layers and affect pore size and morphology, improving adsorption characteristics of the electrolyte at the interface.
Fuel Cells and Microbial Bio-Fuel Cells
BP has never been used in fuel cells. However, given its excellent theoretical specific capacity, BP can be employed for making electrodes of the fuel cells as well as catalyst in the fuel cells. Typically, platinum is used as a catalyst for oxygen reduction reaction in the fuel cells, but it is very expensive and not readily available. Using BP as catalyst or combining BP with Pt as catalytic support can improve the effect of the catalyst as BP has a high surface area and manipulating the layer number can control the surface area significantly.
Extending this applicability, BP can be used as electrodes of microbial bio-fuel cells, which work on the principle of generating electricity from degrading organic substances with the help of microorganisms (bacteria) [16]. Another unexplored application is in enzymatic biofuel cells, where power is generated in-vivo from glucose inside the human body to run small medical devices such as pacemakers [17].
It can be said that this area of research is relatively new and thus can be investigated for cleaner and greener energy generation.
Solar Water Steam Generation
Graphene has been used to covert water into steam using the solar energy and utilizing it for drinkable water collection [18]. It is known that phosphorene has a better phototherapy potential than graphene [19], and thus it can be used for low-cost and efficient drinking water production.
Hydrogen Storage
H2 storage is an important issue for developing hydrogen fuel powered electric vehicles.
Using phosphorene as a material for H2 storage and adsorption is a great idea due to its large SSA and its ability to be stable up to 400°C [20]. Basic applications of BP as a H2 storage material can be for physisorption and chemisorption of molecular hydrogen and addition into metal hydride nanoparticles.
IMPLEMENTATION OF THE NEW APPROACHES FOR ENERGY APPLICATIONS
Supercapacitors
Tuning of the layer number in phosphorene can be done by using different top-down and bottom-up synthesis techniques that have been summarized in the Fig.4.
Manipulation of different process parameters such as suitable precursors, solvents and compatible substrate for CVD is very important for control of layer number and defects. Similarly, disorder in the structure can be achieved by electron-beam irradiation [21] and addition of hybrid materials which can lead to increased SSAs in some cases.
Batteries
Addition of metal oxide nanoparticles such as Fe3O4 (Fig.3) and Mn3O4 in optimal amount can potentially improve battery electrode performance. Essentially, doping concentration follows a Gaussian behavior, thus, selection of optimal concentration is very important. Also, particle size and composition has to be optimized to get the best results.
Fuel Cells
Development of novel catalysts is important for enhancement of cathodic performance of fuel cells. Phosphorene integrated with Pt nanoparticles and carbon nanotubes (CNTs) can be a good idea, given the high cost of traditional Pt catalysts and the high SSA and carrier mobility of phosphorene as well as carbon nanotubes. This process can be extended for usage in polymer electrolyte fuel cells, where phosphorene/Pt/CNT based catalyst can be used. Dispersion of Pt nanoparticles over a phosphorene substrate can lead to increased SSA for catalytic activity and thus improve the oxygen reduction reaction at the electrode.
Solar Water Energy Generation
Phosphorene/Polymer matrix system can be used for developing a solar water energy generator. Using polymers such as polymethylmethacrylate (PMMA) can be used with phosphorene with varying the volume fraction of the phosphorene in order to get the optimal structure and improved performance.
Hydrogen Storage
Incorporation of defects in the phosphorene structure can increase the SSA, which can increase the H2 storage capacity of the material. Another way to increase storage capacity is the decoration of the 2D BP with metal atoms. Also, different BP structures such as nanoribbons, nanowires and quantum dots can be used instead of nanosheets for better performance.
Literature has shown that magnesium hydride (MgH2) being used for H2 storage due to its large capacity and easy availability [22]. Combining MgH2 with phosphorene can be a good alternative to improve the performance at a lower cost.
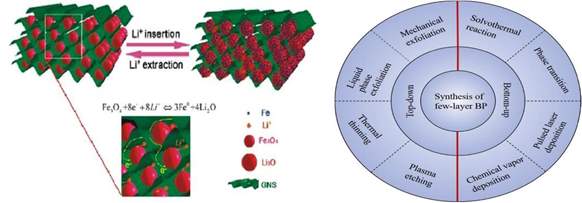
Fig.3 Fe3O4 nanoparticles combined with Fig.4 Overview of BP synthesis techniques Graphene Nanosheets
RESEARCH BENEFITS OF THE NEW APPROACHES
Most of the research that is being done in BP and related technologies for applications in electronics, energy and biomedical sectors has not yet been scaled to commercial market. However, the literature review of BP has revealed that the material has a great potential to excel and become commercialized, especially in the energy storage and generation sector.
In supercapacitors and batteries, BP has a bright future in terms of its superior properties to the existing materials such as graphene, CNTs and TMDs for fabrication of electrodes. Incorporation of the new ideas suggested in this paper can advance the research in SCs and batteries, in terms of improved performance and capacity of the devices and lead to commercialization of BP- based devices for critical energy applications.
Integrating BP in fuel cells has never been done before and thus the idea suggested in this paper, can be a starting point for research in this area. Some of the potential benefits of using BP-based catalysts in fuel cells are increased kinetics of the reactions, leading to better performance and most importantly, reduced dependency on the expensive Pt catalyst, thus cutting down the cost of the system and moving a step towards commercialization.
Using phosphorene for solar water steam generation can significantly reduce the cost of drinking water collection and improve the efficiency of the process.
Advancing research in H2 storage technologies has implications in the automobile industry, specifically in electric vehicles which are the future of cleaner transport. Ideas suggested in this paper for improving performance and reducing cost are a great start towards a cleaner energy generation, not only in the automobile industry, but also in stationary power systems.
REFERENCES
[1] J.-W. Jiang, H. S. Park, Nat. Commun. 2014, 5, 4727
[2] V. Tran, R. Soklaski, Y. Liang, L. Yang, Phys. Rev. B 2014, 89, 235319
[3] E. S. Reich, Nature 2014, 506, 19
[4] F. Xia, H. Wang, Y. Jia, Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics, Nat. Commun. 5 (2014) 4458
[5] F. Xia, H. Wang, Y. Jia, Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics, Nat. Commun. 5 (2014) 4458
[6] J. Qian, X. Wu, Y. Cao, X. Ai, H. Yang, Angew. Chem., Int. Ed .2013, 52, 4633
[7] W. Li, Y. Yang, G. Zhang, Y.-W. Zhang, Nano Lett. 15 (2015) 1691–1697
[8] X. Wang, Y. Chen, O. G. Schmidt, C. Yan, Chem. Soc. Rev. 2016, 45, 1308
[9] J. Yan, T. Wei, Z. Fan, W. Qian, M. Zhang, X. Shen, F. Wei, J. Power Sources 195 (2010) 3041
[10] C. Hao, B. Yang, F. Wen, J. Xiang, L. Li, W. Wang, Z. Zeng, B. Xu, Z. Zhao, Z.Liu, Y. Tian, Adv. Mater. 2016, 28, 3194
[11] T. Lu, Y. Zhang, H. Li, L. Pan, Y. Li, Z. Sun, Electrochim. Acta 55 (2010) 4170
[12] Y. Sun, G. Zheng, Z. W. Seh, N. Liu, S. Wang, J. Sun, H. R. Lee, Y. Cui, Chem 2016, 1,
[13] C. M. Park, H. J. Sohn, Adv. Mater. 2007, 19, 2465
[14] M.C. Stan, J. von Zamory, S. Passerini, T. Nilges, M. Winter, Puzzling out the origin of the electrochemical activity of black P as a negative electrode material for lithium-ion batteries, J. Mater. Chem. A 1 (2013)
[15] G. Zhou, D.-W.Wang, F. Li, L. Zhang, N. Li, Z.-S. Wu, L.Wen, G.Q. Lu, H.-M. Cheng, Chem. Mater. 22 (2010) 5306
[16] D. Pant, G. Van Bogaert, L. Diels, K. Vanbroekhoven, Bioresour. Technol. 101 (2010) 1533
[17] C. Liu, S. Alwarappan, Z. Chen, X. Kong, C.-Z. Li, Biosens. Bioelectron. 25 (2010) 1829
[18] X. Hu, W. Xu, L. Zhou, Y. Tan, Y. Wang, S. Zhu, J. Zhu, Adv. Mater.2017, 29, 1604031
[19] T. Yin, Q. Zhang, H. G. Wu, G. Gao, J. G. Shapter, Y. L. Shen, Q. Z. He, P. Huang, W. Qi, D. X. Cui, NPG Asia Mater. 2017, 9,e383
[20] H. Liu, Y. Du, Y. Deng, P.D. Ye, Chem. Soc. Rev. 44 (2015) 2732
[21] D. Pan, S. Wang, B. Zhao, M. Wu, H. Zhang, Y. Wang, Z. Jiao, Chem. Mater. 21(2009) 3136
[22] Stampfer J Jr, HolleyCJr and Suttle J 1960 1–3 J. Am. Chem.Soc. 82 3504–8
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal



